BOTOX® prevents on average 8 to 9 headache days and migraine/probable migraine days a month (vs 6 to 7 for placebo)
For adults with Chronic Migraine, 15 or more headache days a month, each lasting 4 hours or more. BOTOX® is not approved for adults with migraine who have 14 or fewer headache days a month.
GET PREPARED TO DISCUSS CHRONIC MIGRAINE AND BOTOX® WITH YOUR DOCTOR. HERE ARE SOME IMPORTANT TOPICS TO THINK ABOUT
- HOW MANY DAYS YOU WERE TOTALLY HEADACHE FREE LAST MONTH
- HOW YOUR HEADACHES AND MIGRAINES MAKE YOU FEEL
- HOW HEADACHES AND MIGRAINES AFFECT YOU AND THE PEOPLE YOU KNOW
- WHAT OTHER PREVENTIVE TREATMENTS YOU’VE TAKEN FOR HEADACHES AND MIGRAINES
- HOW OFTEN YOU TAKE ACUTE TREATMENTS (OVER THE COUNTER OR PRESCRIPTION) TO TREAT HEADACHES AND MIGRAINES
- WHETHER BOTOX® IS THE RIGHT OPTION FOR YOU
- ANY OTHER QUESTIONS YOU MAY HAVE ABOUT BOTOX® TREATMENT
BOTOX® DOSING UNITS ARE NOT THE SAME AS, OR COMPARABLE TO, ANY OTHER BOTULINUM TOXIN PRODUCT.
WHAT IS BOTOX®?
BOTOX® is prescription medicine a medical professional injects into muscles to prevent headaches in adults with Chronic Migraine who have 15 or more days each month with headache lasting 4 or more hours each day in people 18 years and older.
It is not known whether BOTOX® is safe or effective to prevent headaches in people with migraine who have 14 or fewer headache days each month (episodic migraine).
WHO SHOULD NOT TAKE BOTOX®?
Do not use BOTOX® if you are: allergic to any of the ingredients in BOTOX® such as botulinum toxin type A and human serum albumin; had an allergic reaction to another botulinum toxin product such as Myobloc® (rimabotulinumtoxinB), Dysport® (abobotulinumtoxinA), or Xeomin® (incobotulinumtoxinA); or have a skin infection at the planned injection site.
WHAT SHOULD I TELL MY DOCTOR BEFORE TREATMENT?
Tell your doctor about all your muscle or nerve conditions, such as amyotrophic lateral sclerosis (Lou Gehrig’s disease), myasthenia gravis, or Lambert-Eaton syndrome, as you may be at increased risk of serious side effects.
INDICATION
BOTOX® is a prescription medicine that is injected to prevent headaches in adults with chronic migraine who have 15 or more days each month with headache lasting 4 or more hours each day in people 18 years or older.
It is not known whether BOTOX® is safe or effective to prevent headaches in patients with migraine who have 14 or fewer headache days each month (episodic migraine).
IMPORTANT SAFETY INFORMATION
BOTOX® may cause serious side effects that can be life threatening. Get medical help right away if you have any of these problems any time (hours to weeks) after injection of BOTOX®:
- Problems swallowing, speaking, or breathing, due to weakening of associated muscles, can be severe and result in loss of life. You are at the highest risk if these problems are pre-existing before injection. Swallowing problems may last for several months.
- Spread of toxin effects. The effect of botulinum toxin may affect areas away from the injection site and cause serious symptoms including: loss of strength and all-over muscle weakness, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice, trouble saying words clearly, loss of bladder control, trouble breathing, trouble swallowing.
There has not been a confirmed serious case of spread of toxin effect away from the injection site when BOTOX® has been used at the recommended dose to treat chronic migraine.
BOTOX® may cause loss of strength or general muscle weakness, vision problems, or dizziness within hours to weeks of taking BOTOX®. If this happens, do not drive a car, operate machinery, or do other dangerous activities.
THINK YOU HAVE CHRONIC MIGRAINE? YOU ARE NOT ALONE
3.2 MILLION U.S. ADULTS ARE LIVING WITH CHRONIC MIGRAINE6,7
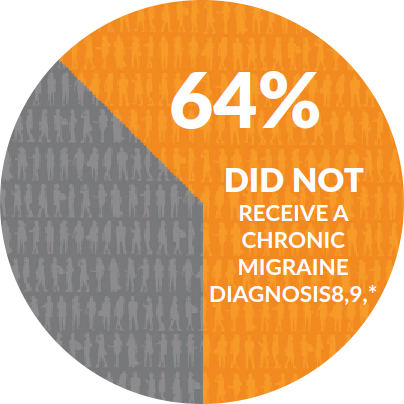
*Based on a study of 200 Chronic Migraine patients who had sought evaluation from a Headache Specialist.
IMPORTANT SAFETY INFORMATION (CONTINUED)
Do not take BOTOX® if you: are allergic to any of the ingredients in BOTOX® (see Medication Guide for ingredients); had an allergic reaction to any other botulinum toxin product such as Myobloc® (rimabotulinumtoxinB), Dysport ® (abobotulinumtoxinA), or Xeomin® (incobotulinumtoxinA); have a skin infection at the planned injection site.
The dose of BOTOX® is not the same as, or comparable to, another botulinum toxin product.